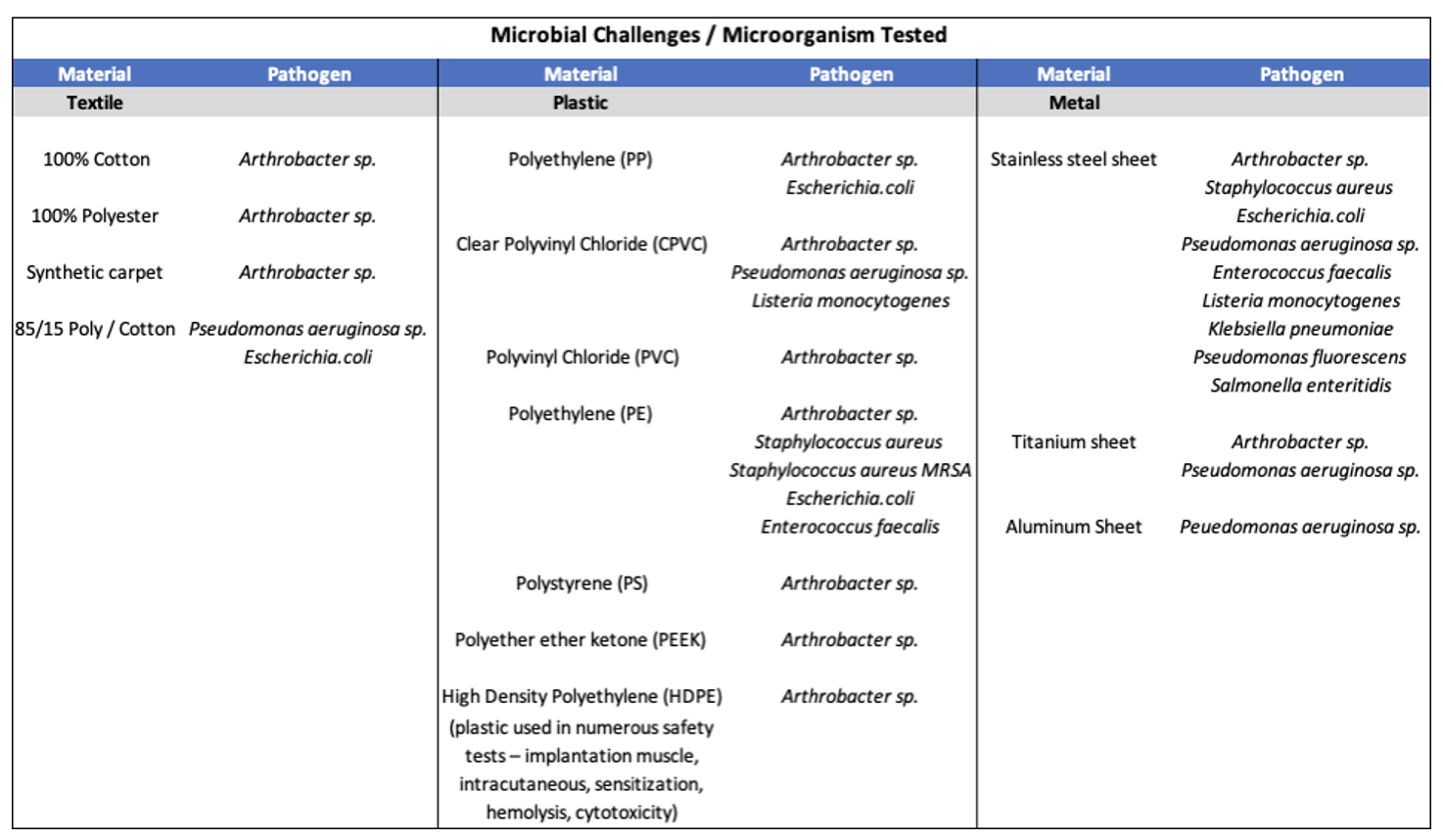
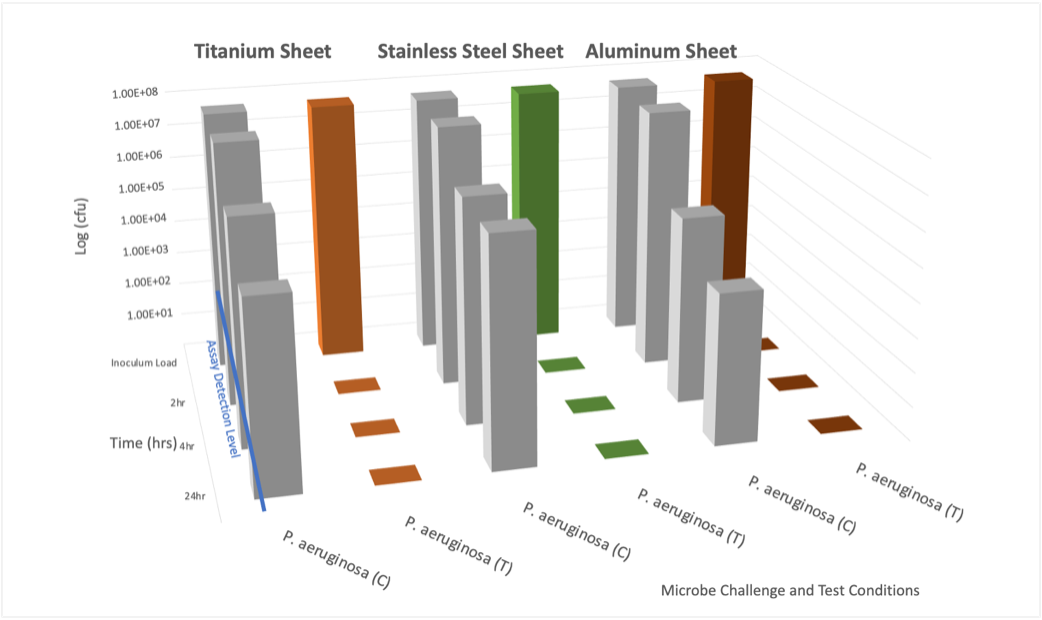
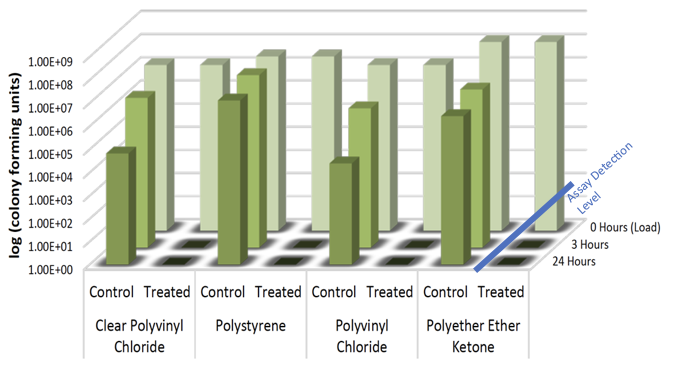
Advanced Antimicrobial Technology
Nano Safe Coatings’ antimicrobial technologies function in ways that enhance the overall performance of the treated surfaces against microorganism attachment and biofilm formation. Uniform, covalent bonding occurs via the unique molecular design and anchored using UV for plastics and heat catalysis for metals. This produces: [1]
- A durable coating of microbe killing nano-networks, that provides mechanical microbial-killing control that meet the needs of this evolving marketplace.
- Avoids the potential negative effects experienced by leaching with other microbe-killing technologies that are available.
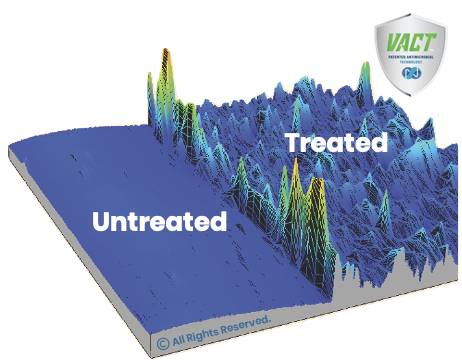
The image illustrates the antimicrobial surface cross-section containing Nano Safe Coatings’ surface bound antimicrobial treatment (VACT™) on the right and an untreated surface on the left. The functionalized surface depicted shows hills representing long chains of stacked Nano Safe Coatings’ antimicrobial coating molecules with an average thickness of 350nm measured with a surface profilometer. (Used with permission from Toronto Metropolitan University, Toronto Canada)